Wednesday, March 08, 2006
Riboflavin Treatment for Keratoconus
Rather than describe the procedure myself I will quote the following article from USAEyes.org
"Keratoconus is a disease of the cornea that makes the cornea become weak and may gradually bulge outward. Most often, this bulging is in the lower half of the cornea and first presents as astigmatism, however not all astigmatism is due to keratoconus. In mild or early stages of keratoconus (forme fruste keratoconus), eyeglasses may correct the astigmatic vision.
The abnormal curvature of the cornea due to keratoconus changes the cornea’s refractive error producing moderate to severe blurriness of vision. As keratoconus advances, rigid gas-permeable (RGP) contact lenses maybe the only non-surgical way to achieve clear vision. If keratoconus continues to advance, scarring of the central cornea may occur.
Approximately half of keratoconus patients have no negative lifestyle effects beyond corrective lenses. The cornea stabilizes after a few years without ever causing severe vision problems. For others, the only resolution to keratoconus has been PKP, with a long healing period and unpredictable refractive error. Even after corneal transplant PKP, keratoconus can reoccur in the new donor cornea. Fortunately, there are two new methods to treat keratoconus that are much less invasive than a corneal transplant.
One new keratoconus treatment is C3-R (Corneal Collagen Crosslinking with Riboflavin) that has been proven to strengthen the weak corneal structure. This method works by increasing collagen crosslinking, which are the natural "anchors" within the cornea. These anchors are responsible for preventing the cornea from bulging out and becoming steep and irregular, consequence of advanced keratoconus.
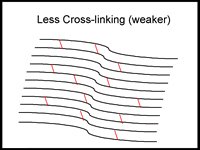
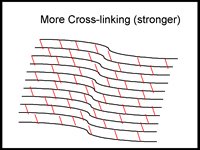
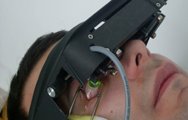
The 30-minute C3-R treatment is performed in the doctor's office. During the treatment, custom-made riboflavin eye drops are applied to the cornea, which is then activated by ultraviolet light. This amazingly simple process has been shown in laboratory and clinical studies to increase the amount of collagen cross-linking in the cornea and strengthen the cornea. In published European studies, such treatments were proven safe and effective in patients.
Another new treatment for keratoconus is Intacs. Intacs are a medical device approved by the FDA for the correction of 1.00 to 3.00 diopters of myopia (nearsighted, shortsighted) and virtually no astigmatism. Intacs inserts are the only refractive surgery procedure that adds structural integrity to the cornea. This unique attribute made Intacs an ideal treatment for keratoconus.
Intacs are clear small semicircular plastic rings of various thickness that are inserted within the cornea at its outer edges. Insertion of these rings flatten the central area of the cornea and correct myopic refractive error. A major advantage of Intacs is that no tissue is removed and there is no ablation or incision across the visual axis.
Intacs surgery is not truly reversible because of the incision, but the Intacs can be completely removed or exchanged for a different size. Intacs inserts cannot be felt by the patient and are no more visible than a contact lens. After insertion and healing, Intacs require no maintenance.
The placement of Intacs inserts remodels and reinforces the cornea, eliminating some or all of the irregularities caused by keratoconus. Follow-up visits will be required to monitor the healing process and to evaluate the visual benefits of the procedure. Even after a successful Intacs procedure for keratoconus, glasses or contacts may be required, however Intacs have been shown to improve vision and reduce or stop the progression of keratoconus, thereby saving the patient from needing PKP.
Intacs have been approved for the treatment of keratoconus by the FDA under a Humanitarian Device Exemption (HDE) Humanitarian Use Devices (HUDs) are medical devices specially designated by the FDA for use in the treatment of fewer than 4000 patients per year with rare medical conditions.
C3-R treatments can be combined with Intacs to flatten the keratoconus cone even more than with Intacs alone. In these cases, C3-R treatments stabilize keratoconus from getting worse as well as help the Intacs reverse the keratoconus steepening that had already occurred."
From what I can see so far this is an exciting development. If the progression can be stopped or even significantly reduced, it will mean that many more keratoconic patients will be able to wear glasses and / or will need less contact lens changes. You will also notice that Intacs have again been mentioned. This is indeed some great stuff. Stay tuned and I hope to post more in the near future. Click to visit my web site for more information about keratoconus
"Keratoconus is a disease of the cornea that makes the cornea become weak and may gradually bulge outward. Most often, this bulging is in the lower half of the cornea and first presents as astigmatism, however not all astigmatism is due to keratoconus. In mild or early stages of keratoconus (forme fruste keratoconus), eyeglasses may correct the astigmatic vision.
The abnormal curvature of the cornea due to keratoconus changes the cornea’s refractive error producing moderate to severe blurriness of vision. As keratoconus advances, rigid gas-permeable (RGP) contact lenses maybe the only non-surgical way to achieve clear vision. If keratoconus continues to advance, scarring of the central cornea may occur.
Approximately half of keratoconus patients have no negative lifestyle effects beyond corrective lenses. The cornea stabilizes after a few years without ever causing severe vision problems. For others, the only resolution to keratoconus has been PKP, with a long healing period and unpredictable refractive error. Even after corneal transplant PKP, keratoconus can reoccur in the new donor cornea. Fortunately, there are two new methods to treat keratoconus that are much less invasive than a corneal transplant.
One new keratoconus treatment is C3-R (Corneal Collagen Crosslinking with Riboflavin) that has been proven to strengthen the weak corneal structure. This method works by increasing collagen crosslinking, which are the natural "anchors" within the cornea. These anchors are responsible for preventing the cornea from bulging out and becoming steep and irregular, consequence of advanced keratoconus.



The 30-minute C3-R treatment is performed in the doctor's office. During the treatment, custom-made riboflavin eye drops are applied to the cornea, which is then activated by ultraviolet light. This amazingly simple process has been shown in laboratory and clinical studies to increase the amount of collagen cross-linking in the cornea and strengthen the cornea. In published European studies, such treatments were proven safe and effective in patients.
Another new treatment for keratoconus is Intacs. Intacs are a medical device approved by the FDA for the correction of 1.00 to 3.00 diopters of myopia (nearsighted, shortsighted) and virtually no astigmatism. Intacs inserts are the only refractive surgery procedure that adds structural integrity to the cornea. This unique attribute made Intacs an ideal treatment for keratoconus.
Intacs are clear small semicircular plastic rings of various thickness that are inserted within the cornea at its outer edges. Insertion of these rings flatten the central area of the cornea and correct myopic refractive error. A major advantage of Intacs is that no tissue is removed and there is no ablation or incision across the visual axis.
Intacs surgery is not truly reversible because of the incision, but the Intacs can be completely removed or exchanged for a different size. Intacs inserts cannot be felt by the patient and are no more visible than a contact lens. After insertion and healing, Intacs require no maintenance.
The placement of Intacs inserts remodels and reinforces the cornea, eliminating some or all of the irregularities caused by keratoconus. Follow-up visits will be required to monitor the healing process and to evaluate the visual benefits of the procedure. Even after a successful Intacs procedure for keratoconus, glasses or contacts may be required, however Intacs have been shown to improve vision and reduce or stop the progression of keratoconus, thereby saving the patient from needing PKP.
Intacs have been approved for the treatment of keratoconus by the FDA under a Humanitarian Device Exemption (HDE) Humanitarian Use Devices (HUDs) are medical devices specially designated by the FDA for use in the treatment of fewer than 4000 patients per year with rare medical conditions.
C3-R treatments can be combined with Intacs to flatten the keratoconus cone even more than with Intacs alone. In these cases, C3-R treatments stabilize keratoconus from getting worse as well as help the Intacs reverse the keratoconus steepening that had already occurred."
From what I can see so far this is an exciting development. If the progression can be stopped or even significantly reduced, it will mean that many more keratoconic patients will be able to wear glasses and / or will need less contact lens changes. You will also notice that Intacs have again been mentioned. This is indeed some great stuff. Stay tuned and I hope to post more in the near future. Click to visit my web site for more information about keratoconus
Comments:
<< Home
Emu
Your comments are welcome. I will quote your comments and reply for all to read in the next week or so.
Post a Comment
Your comments are welcome. I will quote your comments and reply for all to read in the next week or so.
<< Home

